|
A framework to establish the expected effects of climate on forage quantity and quality in a local savanna system was developed to interpret large herbivore population performance patterns in the Kruger National Park. We developed a climate–vegetation response model based on interpretation and synthesis of existing knowledge (literature review) and supported by investigation and analyses of local patterns of climate effects on forage plant performance and chemical composition.Developing the climate–vegetation response model involved three main components, namely (1) defining indicators of forage availability to herbivores (nitrogen productivity, nitrogen quality, carbon-nutrient quality), (2) identifying herbivore species guilds of similar nutritional requirements with respect to these indicators [bulk feeders with tolerance to fibrous herbage (buffalo, waterbuck), bulk feeders with preference for high nitrogen quality forage (short grass preference grazers: blue wildebeest and zebra) and selective feeders where dietary items of relatively high carbon-nutrient quality represented key forage resources (selective grazers: sable antelope, roan antelope, tsessebe, eland)] and (3) developing a process model where the expected effects of plant metabolic responses to climate on key forage resources were made explicit. According to the climate–vegetation response model both shorter-term transient temperature acclimation pulses and longer-term shifts in plant metabolic functionality settings were predicted to have occurred in response to temperature trends over the past century. These temperature acclimation responses were expected to have resulted in transient pulses of increased forage availability (increased nitrogen- and carbon-nutrient quality), as well as the progressive long-term decline of the carbon-nutrient quality of forage. Conservation implications: The climate–vegetation response model represents a research framework for further studies contributing towards the enhanced understanding of landscape-scale functioning of savanna systems with reference to the interplay between climate, vegetation and herbivore population dynamics. Gains in such understanding can support sound conservation management.
Herbivore population trends in the Kruger National Park (KNP) over the past century were traditionally explained with reference to decimation by hunting and epizootics (Joubert 2007a; Pienaar 1963), effects of rainfall (Dunham, Robertson & Grant 2004; Owen-Smith & Ogutu 2003; Whyte & Joubert 1988) and predation (Harrington et al. 1999; Joubert 2007a, 2007b; Owen-Smith, Mason & Ogutu 2005; Owen-Smith & Mills 2006; Owen-Smith & Mills 2008). However, the long-term and spatially widespread nature of population trends suggested that over-arching landscape-scale influences may also be involved to an as yet unknown extent. A greater role of climate effects as a long-term landscape-scale factor was therefore implicated. Accordingly, we initiated a study to explore the extent to which climate–vegetation responses could plausibly explain diverse and divergent spatiotemporal patterns in population performance of eight large herbivore species, namely African buffalo (Syncerus caffer), blue wildebeest (Connochaetes taurinus), plains zebra (Equus burchelli), waterbuck (Kobus ellipsiprymnus), sable antelope (Hippotragus niger), roan antelope (Hippotragus equinus), tsessebe (Damaliscus lunatus lunatus) and eland (Taurotragus oryx). To interpret large herbivore population performance patterns over space and time in relation to climate it was necessary to develop a framework in which the expected effects of climate on forage quantity and quality were established. Towards this purpose we developed a climate–vegetation response model. This model was developed through interpretation and synthesis of existing knowledge (literature review) and supported by analyses of local patterns of climate effects on forage plant performance and chemical composition. Forage availability to herbivores was taken to be a function of plant productivity and the nutritional quality of the resultant plant material (Fryxell 1991; Holdo, Holt & Fryxell 2009), based on concentrations of protein (nitrogen), non-structural carbohydrates (TNC) and minerals in plant tissues. Key resources for herbivores thus relate to nitrogen productivity, nitrogen quality and carbon-nutrient quality. A central notion of the climate–vegetation response model is that levels of these three forage resource indicators are affected differentially by plant metabolic processes in response to the nature of edaphoclimatic resources (water, temperature and nutrients) available to plants. To the extent that different herbivore species have divergent nutritional requirements in respect of these key forage resources, we expected that their population performances would be affected differentially by conditions of the edaphoclimatic environment (spatial scale) and climate (temporal scale). The climate–vegetation response model developed here accordingly consists of three main components: • definition of indicators of forage availability to herbivores (nitrogen productivity, nitrogen quality and carbon-nutrient quality)
• identification of herbivore species guilds of similar nutritional requirements in respect of these indicators
• development of a climate–plant metabolic response process model where the expected effects of plant metabolic responses to climate on key forage resources are made explicit. With the climate–vegetation response model as explanatory framework, large herbivore population performance patterns in both space and time in the KNP were investigated and interpreted (Seydack et al. 2012). In a broader sense, however, our climate–vegetation response model is intended to represent a research framework for further studies contributing towards the enhanced understanding of landscape-scale functioning of savanna systems in respect of the interplay between climate, vegetation and herbivore population dynamics.
Study area
The KNP is situated in north-eastern South Africa and represents a large (approximately 2 000 000 ha) semi-arid savanna system. The area lies between the Drakensberg escarpment in the west and the Mozambique coastal plain in the east. Covering an altitudinal range of between 200 m a.s.l. and 700 m a.s.l., the KNP falls within two disparate climate zones as defined by the South African Weather Service (Venter, Scholes & Eckhardt 2003). The area north of the Olifants River is in the northern arid bushveld zone, receiving 300 mm – 500 mm rain per year, whilst the southern part of the park falls into the lowveld bushveld zone, with an average annual rainfall of 500 mm – 700 mm per year. Years of pronounced below-average rainfall were 1963–1964, 1973, 1983–1984, 1987, 1991–1992 and 1998 (Figure 1). Growing season duration (v. Schulze 1997) is on average about 1.5 months shorter in the north than in the south (96 days compared with 142 days, respectively).
 |
FIGURE 1: Mean annual rainfall in the Kruger National Park (1954¢2006).
|
|
Temperature records from stations within or near the KNP followed trends congruent with those recorded globally (Figures 2 and 3). Three phases of temperature increases were discernable. The first reached peak values between 1937 and 1947, followed by a second phase of increasing temperature towards the period 1958–1960 and a third, still ongoing, warming phase which commenced between 1975 and 1978. Locally differentiated temperature trends are shown in Figure 3. Regional temperature lapse rates for monthly means of daily maximum and minimum temperatures are –0.777 °C×100 m-1 and –0.465 °C×100 m-1, respectively (Schulze 1995). As indicated by higher summer-to-winter heat unit differentials (determined according to Schulze 1997), northern areas of the park experience more pronounced temperature seasonality.
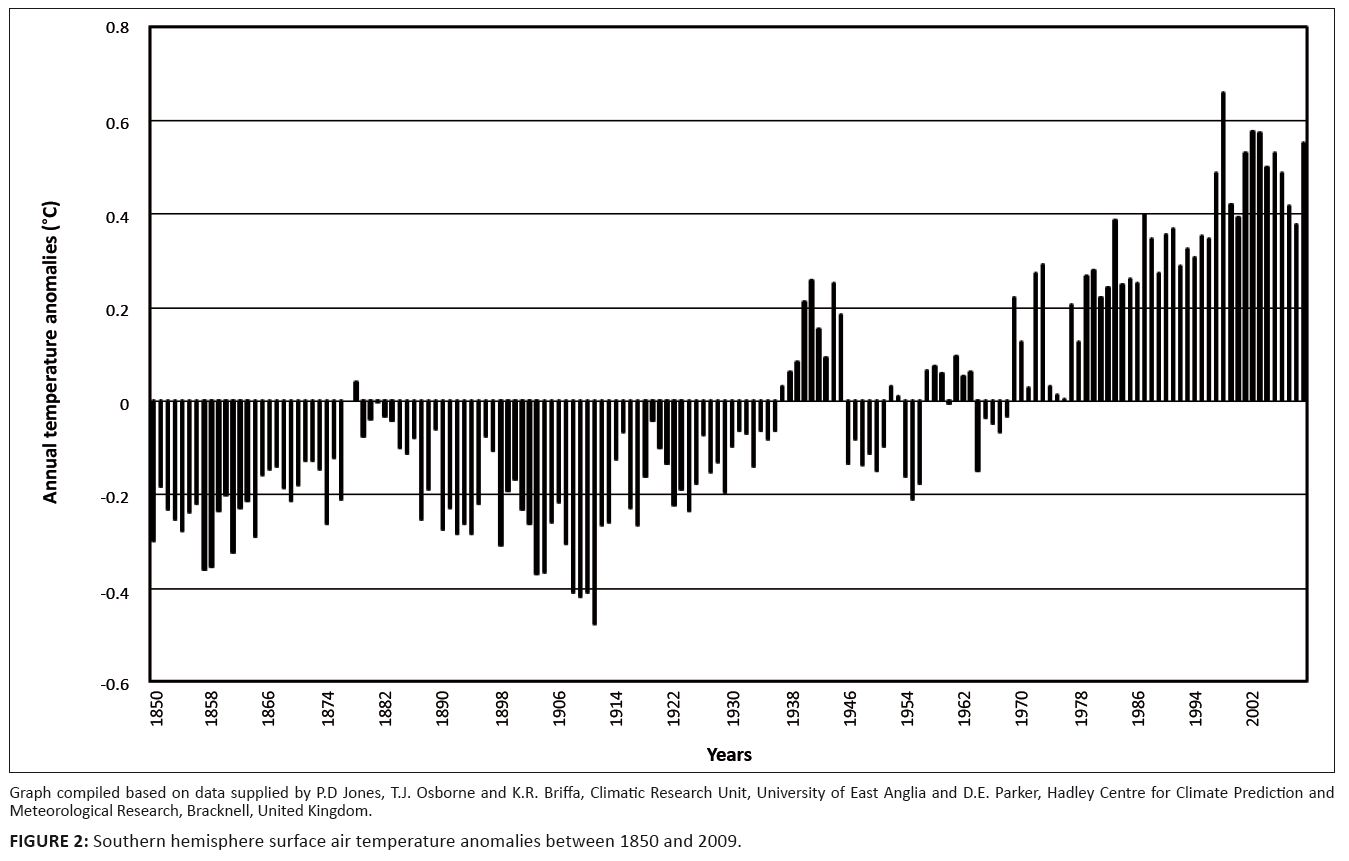 |
FIGURE 2: Southern hemisphere surface air temperature anomalies between 1850 and 2009.
|
|
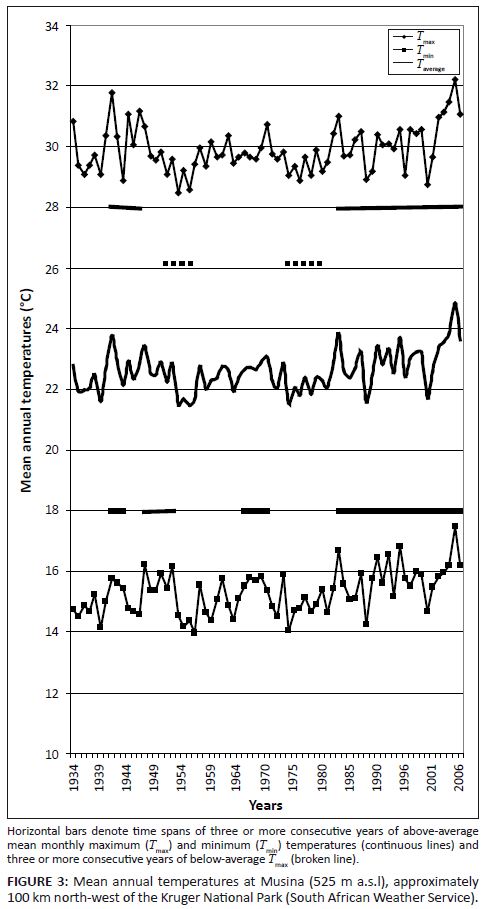 |
FIGURE 3: Mean annual temperatures at Musina (525 m a.s.l), approximately
100 km north-west of the Kruger National Park (South African Weather Service).
|
|
The park is longitudinally divided into granitic substrates forming relatively nutrient-poor sandy soils in the west and more nutrient-rich, basalt-derived clay soils in the east (Venter et al. 2003). The savanna vegetation on nutrient-poor substrates tends to be dominated by trees of Combretaceae (e.g. Combretum and Terminalia species) and Caesalpineaceae, whereas those on more nutrient-rich clay soils are dominated by trees of Mimosaceae (especially Acacia species) (Gertenbach 1983; Venter et al. 2003). The vegetation of the northern KNP is characteristically dominated by mopane (Colophospermum mopane) and this species is well represented in broad-leaved bushveld vegetation types on granites and broad-leaved shrubveld associated with basalts (Gertenbach 1983; Venter et al. 2003). The herbaceous layer of the KNP is dominated by C4 grass species (Kennedy, Biggs & Zambatis 2003) and the more nutrient-rich savanna vegetation types on clay soils carry dense stands of nutritious, high-bulk grasses (Venter et al. 2003).
Development of an explanatory framework
Exploration of spatiotemporal patterns in population performance of eight large herbivore species revealed three population performance response groups (i.e. groups of large herbivore species that shared broad-scale habitat preferences and similar population performance trends over time) and group-specific correlations with climate variables (Seydack et al. 2012). We developed an explanatory framework – the climate–vegetation response model – which permits the interpretation of these correlative patterns in relation to climate effects. As part of the climate–vegetation response model we (1) defined indicators of forage availability (quantity and quality), (2) established forage selection requirements characteristic of and unique to the three herbivore population performance response groups (nutritional guilds), and (3) developed a plant-climate response process submodel. The resulting climate–vegetation response model defined the expected effects of climate on indices of forage availability, thereby providing an explanatory framework for the interpretation of spatiotemporal patterns in herbivore population performance in relation to climate. The model was developed largely based on the interpretation and synthesis of relevant literature. Some results of local research contributed towards the definition of key forage resource indices.
Definition of indicators of forage quantity and quality
Leaf and stem material of the grass species Panicum maximum, Panicum coloratum and Themeda triandra were collected in and beyond the roan enclosure (Nwashitshumbe, northern KNP) during September 2007, November 2007, August 2008 and February 2009. The apical 20 cm of grass stems were collected as samples of stem material. The samples originated from a study to explore the effects of fire frequency on forage quality, where sampling was undertaken in four treatment blocks (three inside and one outside of the enclosure) defined by the time since the last burn. Each treatment block contained three sampling localities (replications) to represent a total of 12 samples per sampling event. The collected grass material was dried at 55 °C overnight and mill ground through a 1-mm sieve to form homogeneous powdered samples. The concentrations of ash minerals and TNC were determined, as well as in vitro organic matter digestibility (OMD) of the powdered sample material. These analyses were performed by the Analytical Services department of the Agricultural Research Council (ARC) in Irene. Ashing was performed in a furnace at 600 °C. TNC were analysed as reducing sugars after complete enzymatic hydrolysis to monosaccharides. The method entailed gelatinisation of all starch in the sample by autoclaving, followed by enzymatic hydrolysis of the starch to glucose and determination of glucose content by spectrometric measurement. Duplicate results were within 3% of the mean for each pair. In vitro OMD was determined following an adaptation of the two-phase technique described by Tilley and Terry (1963), with some modification introduced by Engels and Van der Merwe (1967). It involved a 24-hour-long fermentation by rumen micro-organisms in a buffer solution followed by a 48-hour-long pepsin digestion after acidification. The reduction in the organic matter content was ascribed to digestion of the sample. For the determination of d13C and d15N, samples were combusted in an automated Thermo1112 Elemental Analyser (Carlo Erba, Milan). The resultant CO2 and N2 gases were then introduced to a Thermo Delta stable light isotope XP mass spectrometer via a continuous flow-through inlet system (Thermo Conflo III). 13C/12C and 15N/14N ratios were expressed in the delta (d) notation in parts per thousand (o/oo) relative to international standards (Pee Dee Belemnite standard for carbon; N2 air for nitrogen). Standard deviations of repeated measurements were less than 0.1o/oo for d13C (Stable Light Isotope Unit, Department of Archaeology, University of Cape Town). Grass sample collections during November 2007 and February 2009 represented spring and summer sampling events, respectively, and plant growth under conditions of concurrent high levels of temperature and water availability (TW conditions). Dry-season grass and stem material collected during September 2007 had been subject to summer greening in November–December 2006 (TW metabolic cueing at sub-mature stages; mean monthly maximum and minimum temperatures at 33.9 °C and 21.1 °C, respectively) and water-stressed summer growth conditions (Tw growth realisation at mature stages). Dry-season material collected in August 2008 represented conditions following spring greening in September–October 2007 (tw metabolic cueing at sub-mature stages; mean monthly maximum and minimum temperatures at 30.6 °C and 17.7 °C, respectively) and summer growth subject to high rainfall conditions (TW growth realisation at mature stages). The main objective of this part of the study was to determine how these varying growth conditions affected the chemical composition and digestibility of the grass tissues and the implications for key indices of forage quality defined in the context of this study.
Climate–vegetation response model
Indicators of forage availability
Nitrogen productivity: Nitrogen productivity is defined as the quantitative availability of forage items for herbivores of a given nitrogen quality (plant protein content) as a result of biomass production. Grass productivity is known to be strongly promoted by water availability [positively correlated with rainfall (Zambatis 2004)] and temperature, especially in the case of tropical grass species (Long 1999; Luo 2007; Wan et al. 2005), which have high temperature optima for growth (Dwyer et al. 2007; ’t Mannetje 1982; Tudsri, Matsuoka & Kobashi 2002; Wilson & Ford 1971). Warming-induced increases in biomass production of C4 grasses have been demonstrated under experimental conditions (An et al. 2005; Bijoor et al. 2008). Accordingly, nitrogen productivity of C4 grasses and subtropical woody vegetation is expected to increase over time as ambient temperature increases (Figure 2). However, at progressively higher nitrogen productivity, an inverse relationship to nitrogen quality due to nitrogen dilution is generally encountered (An et al. 2005; Fryxell 1991; Fryxell et al. 2005; Mutanga et al. 2004; Wilson 1982).Nitrogen quality: High nitrogen quality of forage implies high nitrogen concentrations of plant tissues; that is, plant protein content not unduly diluted by structural or non-structural carbon and of relatively high digestibility. An index of nitrogen quality is defined here as the product of nitrogen concentration and OMD of the plant tissues involved. Wet-season nitrogen quality tended to be higher early in the wet season (spring) compared with later in the wet season (summer) (Figure 4c). Nitrogen content in summer leaf material (SUL) was diluted by structural carbon relative to that of spring (SPL) leaf material (analysis of variance for carbon content, followed by a post hoc Bonferroni test showed SUL > SPL; p < 0.00001) and carbon-nutrient levels (Figure 4d: SUL > SPL). This is congruent with nitrogen dilution through growth under conditions of relatively high temperature and water availability (TW conditions), as
typically experienced by tropical grasses (Wilson & Ford 1971).
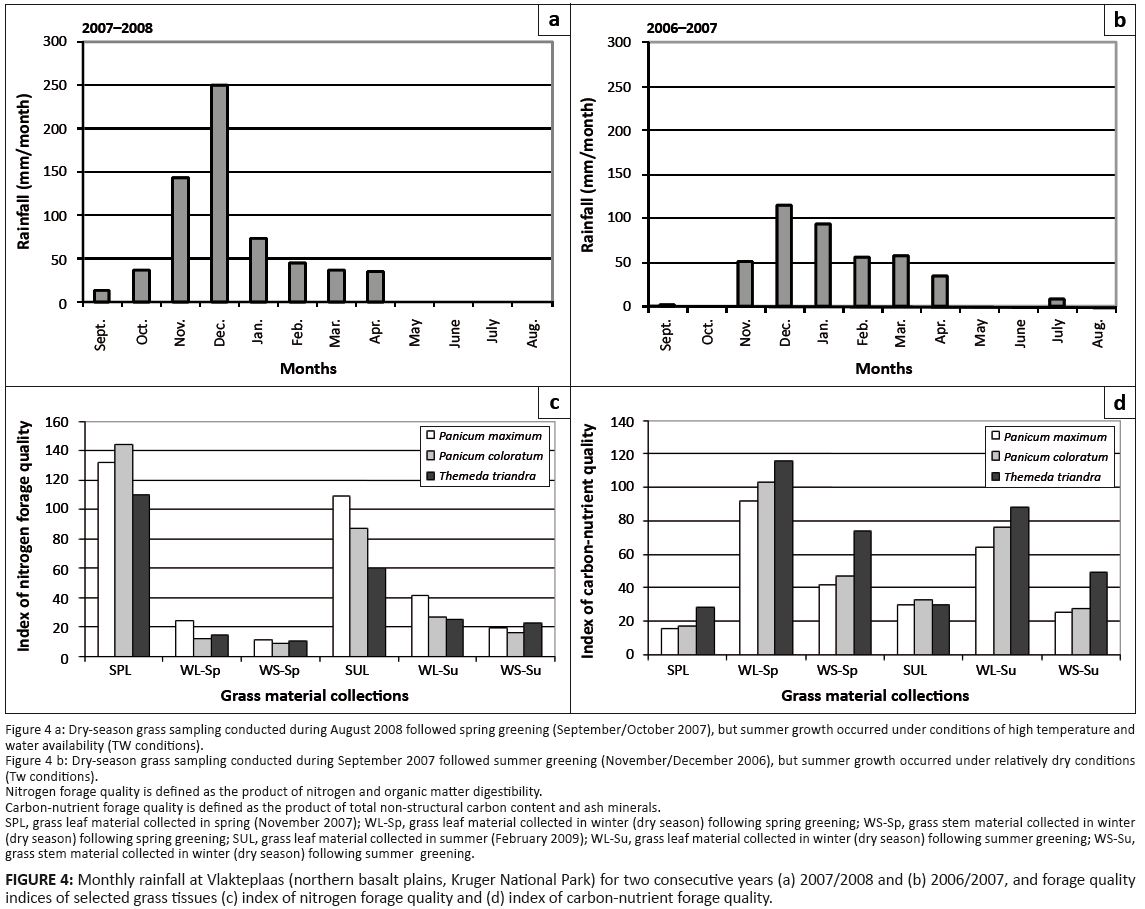 |
FIGURE 4: Monthly rainfall at Vlakteplaas (northern basalt plains, Kruger National Park) for two consecutive years (a) 2007/2008 and (b) 2006/2007, and forage quality
indices of selected grass tissues (c) index of nitrogen forage quality and (d) index of carbon-nutrient forage quality.
|
|
Dry-season leaf (WL) and stem (WS) material were sampled to represent two sets of conditions (Figure 4): summer greening and water-stressed summer growth (WL-Su/WS-Su) and spring greening and summer growth with high rainfall (WL-Sp/WS-Sp), respectively. Nitrogen quality of dry-season WL and WS material (Figure 4c) was enhanced following water-stressed summer growth (WL-Su/WS-Su: Figure 4b; Tw conditions indicated by low d15N values) compared with such material following summer growth in higher rainfall conditions (WL-Sp/WS-Sp: Figure 4a; TW conditions indicated by high d15N values; N-quality: leaf material: F = 113.36, n = 72, p < 0.000001; stem material: F = 50.77, n= 72, p < 0.000001). OMD of dry-season grass tissue (both leaf and stem material) following water-stressed summer growth (Tw conditions; Figure 4b) was significantly higher than of dry-season material following summer growth under TW conditions (Figure 4a; OMD: ANOVA: Bonferroni post hoc test: WL-Su > WL-Sp; p < 0.0001; WS-Su > WS-Sp:p < 0.00001). OMD of dry-season grass leaf material was positively related to nitrogen content and negatively correlated with d15N values (multiple regression of OMD on N and d15N: ß of N = +0.758; ß of d15N = –0.348, R2 = 0.42, n = 72). According to the results of Codron et al. (2005) seasonal shifts in plant d15N corresponded positively with seasonal summer rainfall, supporting our interpretation that d15N values index summer growth activity under TW conditions. These relationships are consistent with general findings that metabolic activity under water stress promotes digestibility (Wilson 1982) and that high growth activity under TW conditions results in reduced forage quality. Metabolic activity under conditions of high temperature and water availability (TW conditions) is expected to negatively affect dry matter digestibility as it promotes more rapid growth and stem development of, particularly, tropical grasses (’t Mannetje 1975; Wilson 1982; Wilson, Taylor & Dolby 1976). High growth temperatures also hasten the maturation of individual tissues and the digestibility of plant tissue is usually higher when grown at low temperatures, such as in spring or winter (’t Mannetje 1984; Wilson 1982).Carbon-nutrient quality: Carbon-nutrient quality of forage items is defined here as the product of TNC and ash mineral concentrations (Figure 4d). Dry-season TNC content of grass leaf material following spring greening was significantly higher than that of leaf material following summer greening (dry-season TNC content: WL-Sp > WL-Su; F = 25.02, p < 0.00001). A similar pattern was found for ash (mineral) contents (ANOVA, followed by post hoc Bonferroni test: WL-Sp > WL-Su and WS-Sp > WS-Su, p < 0.00001). These results are consistent with a metabolic response mode of low-temperature growth during spring (tw metabolic cueing at sub-mature stages) resulting in storage allocation of TNC (Estiarte & Peñuelas 1999; Herms & Mattson 1992; Wilson 1975) and of minerals (Balasko & Smith 1971). In accordance with these findings, high carbon-nutrient quality was typically
encountered in dry-season leaf material (Figure 4d) and was higher in dry-season leaf and stem tissue in grass cohorts that had been subject to spring greening (Figure 4d; ANOVA followed by a post hoc Bonferroni test: WL-Sp > WL-Su, p < 0.00001; WS-Sp > WS-Su, p < 0.00001). Carbon-nutrient quality forage items are thus characterised by relatively high concentrations of TNC and ash minerals resulting from substantial metabolic allocation to storage under circumstances promoted by constrained growth (particularly owing to low temperatures, but also moisture stress: tw conditions) but sustained photosynthesis. Carbon-nutrient quality of dry-season plant leaf and stem material of T. triandra (Andropogoneae) was generally higher than for the two species of Paniceae (Figure 4d; ANOVA and post hoc Bonferroni tests: p < 0.01). This reflected the predisposition of andropogonoid species, geared to cope with more pronounced seasonality (Osborne 2008), towards greater metabolic scope for storage investment. The typical accumulation of tannin-like substances in the foliage of Andropogoneae indicates a carbon excess metabolism with pronounced allocation to storage (Baas 1989; Herms & Mattson 1992). Such physiological characteristics are considered adaptive to ensure sustained metabolic performance under seasonally variable conditions of resource availabilities (temperature and water: tW/Tw/tw conditions). All species of Andropogoneae function according to the NADP-ME biochemical subtype and these are implicated to operate at lower temperature optima than species of the other subtypes, including, inter alia, species of Paniceae (Ehleringer, Cerling & Helliker 1997). Grass species of this biochemical subtype, and particularly of Andropogoneae, also exhibited the smallest responses to changes in environmental conditions (Buchmann et al. 1996). These findings are suggestive of the capacity of Andropogoneae species for sustained metabolic performance at variable and lower resource levels (dry-season growth), underpinning their metabolic scope for storage of metabolites (TNC and minerals for enhanced carbon-nutrient quality).
Herbivore species guilds of key nutritional requirements
In this section we present evidence suggesting that herbivore species within particular population performance response groups share similar broad-scale forage selection requirements. Buffalo and waterbuck are considered roughage feeders able to digest fibrous food relatively efficiently (Hofmann 1973, 1989). Despite the relatively unselective bulk grazing habit of buffalo, they adjust their feeding patterns in relation to seasonally changing forage quantity and quality (Macandza, Owen-Smith & Cross 2004). However, for both these species food quantity seems to override exacting quality requirements. This is in contrast to blue wildebeest and zebra, which have clear preferences for short-grass areas on nutrient-rich soils (Grange & Duncan 2006; Traill 2004). Both species select grass patches of high nitrogen quality for feeding, although zebra, as a hindgut fermenter, can tolerate more fibrous and longer-grass feeding conditions (Bodenstein,
Meissner & Van Hoven 2000; Smuts 1972).Apart from species-specific, often seasonally changing, feeding preferences (Dunham et al. 2004; Heitkönig & Owen-Smith 1998; Joubert & Bronkhorst 1977; Magome et al. 2008; Watson & Owen-Smith 2000), all four selectively feeding species (i.e. tsessebe, eland, sable and roan antelope) are able to adapt to a wide range of grass heights and often include medium to tall grasses in their diets (Joubert 1976; Skinner & Chimimba 2005; Traill 2004). According to a study by Parrini (2006) sable antelope do not avoid stemmy grass tufts and include tall stemmy species generally avoided by short-grass grazers. Sable antelope were found to be particularly tolerant of stems in habitats with intermediate to high nutrient levels. Heitkönig and Owen-Smith (1998) found a similar pattern in roan antelope, which tolerated stems when leaf quality was high. The preferred feeding sites of tsessebe typically have soils with a high clay content (Dörgeloh 2006). Topi (Damaliscus lunatus jimela), with foraging habits believed to be similar to those of tsessebe, selected for the tallest plants and species with relatively long leaves when feeding on grass with more than 20% green leaves (Duncan 1975 op. cit. Parrini 2006). These patterns of ingesting stem material together with leaf matter and the relatively high importance of nutrient contents of these are commensurate with the maintenance of relatively high dietary TNC and mineral nutrient levels resulting in carbon-nutrient ratios as required for sustained carbon-nutrient quality. Numerous studies have revealed the prominence of andropogonoid grass species in the preferred habitat and forage species assemblages of sable antelope (Le Roux 2010; Magome 1991; Parrini 2006), roan antelope (Joubert 1976; Van Lavieren & Esser 1979), tsessebe (Gureja & Owen-Smith 2002) and eland (Watson & Owen-Smith 2000). As indicated in the previous section, andropogonoid grass species would tend towards a sustained metabolic performance type metabolism and thus have a propensity towards carbon and nutrient storage, particularly when subject to seasonally low temperature conditions. Under such metabolically favourable conditions, these species are expected to have high carbon-nutrient quality; that is, a combination of favourable levels of TNC, nitrogen and minerals. All these features of forage selectivity, including tolerance of stemminess, prominence of andropogonoid grass species, adaptability to a wide range of grass heights and, in the case of browsing, feeding on freshly
fallen TNC-rich leaves [see Skinner & Chimimba (2005) for reference to eland] indicate that these species can be classed as selective feeders of plant parts of relatively high carbon-nutrient quality. In summary, the eight species studied here are grouped into three guilds of nutritional requirements: bulk feeders with tolerance to fibrous herbage (buffalo and waterbuck), bulk feeders with preference for high nitrogen quality (short-grass grazers: blue wildebeest and zebra) and selective feeders for which dietary items of relatively high carbon-nutrient quality are of particular importance (selective grazers: sable antelope, roan antelope, tsessebe and eland).
Plant–climate response processes
Plant metabolic performance trade-off relationships: Owing to resource limitations plants are subject to trade-offs between growth, maintenance, storage, reproduction and defence (Herms & Mattson 1992). Consequently, there are, inter alia, strong inverse relationships between the functional allocation to growth and non-growth processes, notably carbon storage (Herms & Mattson 1992). Accordingly, there is an allocation trade-off between quantity of plant tissue produced through growth (structural plant content) and quality, represented by the proportion of non-structural plant content (metabolically active components and storage products). Thus, abundance of resources such as water, nutrients and temperature enhance productivity, but increasing productivity then progressively reduces plant tissue quality for herbivores through nutrient dilution (An et al. 2005; Dwyer et al. 2007; ’t Mannetje 1982, 1984; Tudsri et al. 2002; Van Soest, Mertens & Deinum 1978; Wilson & Ford 1971; Wilson 1982), reduction in digestibility (’t Mannetje 1975, 1984; Van Soest et al. 1978; Wilson & Ford 1973; Wilson & ‘t Mannetje 1978) and reduced allocation to storage (low TNC content) (Estiarte & Peñuelas 1999; Herms & Mattson 1992; Van Soest et al. 1978).The climate–vegetation response model was developed to integrate existing knowledge and to form part of an explanatory template for spatiotemporal patterns of climate–vegetation response processes controlling forage quantity and quality. At the core of this model is the metabolic performance trade-off model, from which predictions can be made regarding divergent plant responses to site and climate-linked resource constraints and their impact on parameters of forage availability to herbivores. The principal trade-off in the context of this model is between maximised peak performance, closely tracking and responsive to conditions of resource surplus (RMP; resource responsive metabolic performance mode), and sustained performance when subject to conditions of varying resource levels or deficits (SMP; sustained metabolic performance mode). The capacity for maximum metabolic performance under conditions of resource surplus is at the cost of the capacity for sustained performance under conditions of resource deficits or fluctuations. In line with this reasoning, we expect a propensity towards the RMP mode under conditions of high and less fluctuating levels of temperature and water availability (TW conditions: combination of relatively high and sustained levels of water and temperature during the growing season), whereas under conditions of fluctuating resource levels and pronounced deficits (regarding temperature and water; tW or Tw conditions), the SMP mode would be expected to predominate. High peak-level performance characteristics of the RMP mode require high turnover metabolism (capacity for high instantaneous rates of photosynthesis; Amax). This is generally associated with high foliar nitrogen levels (Lambers, Chapin & Pons 1998) resulting in increased nitrogen/carbon ratios of plant tissues. In contrast, sustained performance capacity of the SMP mode requires resource use efficiency and is associated with the propensity towards somatic accumulation [size, storage, longevity: relatively high carbon/nitrogen ratios of plant tissues; Herms and Mattson (1992)]. Furthermore, stress-tolerant plants (SMP mode) tend to allocate an especially high proportion of available energy to maintenance respiration required to tolerate adverse environmental conditions (Taylor 1989). Plants in a relative SMP mode are accordingly expected to be even more sensitive to resource conditions inducing elevated maintenance respiration, such as increased mean mimimum temperature (reflecting nocturnal temperature conditions) (Tmin). Resource constraints on growth are generally more intense than their inhibitory effect on photosynthesis. As a consequence of carbon acquisition exceeding growth requirements, the products of photosynthesis accumulate, primarily as carbohydrate storage products (Baas 1989; Herms & Mattson 1992; Lambers & Poorter 1992). When tracking resource surplus conditions favourable for both photosynthesis and growth in RMP-mode plants, the resource-level response relationships for growth and photosynthesis tend to be similar (Wilson & Ford 1973). In SMP-mode plants, stress tolerance capacity is associated with photosynthesis being relatively less sensitive to resource levels than growth. Accordingly, there is greater scope for source (photosynthesis) to sink (growth) imbalance in SMP-mode plants, favouring allocation to secondary metabolism and storage as carbon assimilation levels exceed those which are required for growth. Plants in relative SMP mode are thus comparatively stress tolerant in order to cope with fluctuations in resource levels. As such their metabolism is capable of functioning at relatively low temperature and water availability levels/optima (broad metabolic amplitude; SMP-tw functional efficiency). Such metabolic capacity is, however, associated with relatively high storage-to-growth allocation priority and comparatively high maintenance respiration costs (i.e. temperature sensitivity). In contrast, plants in relative RMP mode are geared to the maximum use of resource surplus conditions, functioning at relatively high temperature and water resource level optima (narrow metabolic amplitude) and high growth-to-storage priority (Figure 5).
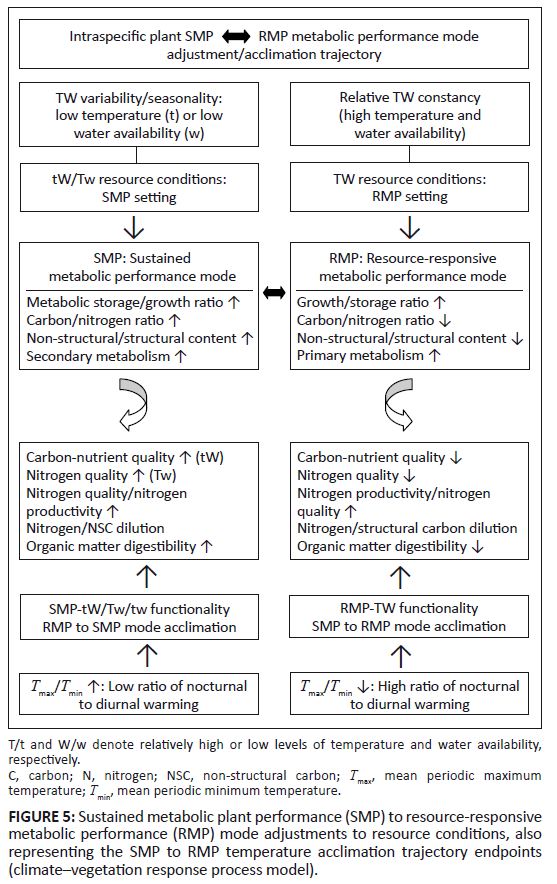 |
FIGURE 5: Sustained metabolic plant performance (SMP) to resource-responsive
metabolic performance (RMP) mode adjustments to resource conditions, also
representing the SMP to RMP temperature acclimation trajectory endpoints
(climate¢vegetation response process model).
|
|
Plant metabolic performance mode settings: The relative expression of SMP to RMP mode functionality/acclimation is a function of the combined availability levels of temperature and water (TW resource conditions). The higher the concurrent and uninterrupted levels of temperature and water available to plants, the more pronounced the intraspecific metabolic adjustment towards higher RMP mode functionality settings. The relative expression of SMP or RMP depends on the spatiotemporal nature of resource availability for plants (water and temperature resource levels and variability or seasonality), shaping forage quantity and quality as a consequence of the relative prevalence of SMP-tW/Tw to RMP-TW functionality (Figure 5). Resource conditions in the northern KNP are characterised by greater moisture stress levels and higher degrees of resource level fluctuations regarding both moisture availability and temperature compared to the southern KNP (increasing north to south rainfall gradient: KNP-north = 483 mm/year; KNP-central = 535 mm/year; KNP-south = 632 mm/year). Growth season duration (v. Schulze 1997) is, on average, about 1.5 months shorter in the northern KNP. According to the metabolic performance trade-off model, we therefore expect the SMP mode to predominate (intraspecifically) in northern KNP areas and the RMP mode to be more prevalent in southern areas of the park. Conditions prevailing in the central KNP are expected to promote an intermediate position in this regard. Conditions for plant functionality are also expected to change along an altitudinal gradient [temperature lapse rates for monthly means of daily maximum and minimum temperatures are –0.777 °C×100 m–1 and –0.465 °C×100 m–1, respectively (Schulze 1995)]. A pattern of lower temperatures and increased rainfall at higher altitudes represents tW conditions, with Tw conditions prevailing at lower altitudes. Combining the north–south water availability and altitudinal temperature gradients, plant functionality settings were identified and the associated expected implications for forage quality indicated in accordance with the metabolic performance trade-off model (Table 1).
|
TABLE 1: Plant functionality settings according to prevailing environmental conditions in various parts of the Kruger National Park and their expected consequences for
forage quality (climate¢vegetation response model).
|
As a general notion, nitrogen quality is usually considered to be higher in plants on nutrient-rich geological substrates, resulting in higher palatability for herbivores (Skidmore et al. 2010; Venter et al. 2003). This pattern may, however, be modified at various spatial scales by the availability of water (Mutanga et al. 2004) and factors resulting in nitrogen dilution (accentuated SMP or RMP functional expression as in northern and southern KNP, respectively). At a spatial scale we thus generally expect the prevalence of relatively high nitrogen quality forage on nutrient-rich geological substrates in the central KNP (Table 1). Temperature acclimation and forage quality: Ambient temperature has been increasing over the past century (Figure 2). In particular, Tmin, which represents nocturnal temperature conditions, has increased more steeply than the mean periodic maximum temperature (Tmax), which represents diurnal temperature conditions (Houghton et al. 1996). Increasing Tmin is expected to stimulate nocturnal maintenance respiration (Wan et al. 2009), promoting temperature acclimation in order to restore the original steady-state respiration-to-photosynthesis ratio (Dewar, Medlyn & McMurtrie 1999). As temperature increases, leaf metabolic pools adjust until the original steady-state respiration-to-photosynthesis ratio is re-established. Leaf labile carbon, starch and protein pools decrease in response to increased temperature, eventually reaching a new, lower steady state, consistent with the general pattern of plant carbohydrate and soluble protein content being negatively correlated with growth temperature (Dewar et al. 1999; Wan et al. 2009). These acclimation responses represent intraspecific SMP to more RMP functionality shifts (SMP-to-RMP temperature acclimation). This has consequences for forage quality, such as reduced carbohydrate and mineral storage levels (progressively lowered carbon-nutrient quality) and reduced nitrogen quality due to nitrogen dilution and lowered digestibility resulting from high RMP mode-based growth rates (Figure 5). In the climate–vegetation response model the expression of relative RMP to SMP functionality results from, inter alia, the acclimation to changing temperature conditions. In response to increasing Tmax (daytime temperatures), the productivity of tropical and subtropical forage plants (especially C4 grasses) is increased (Long 1999; Luo 2007; Wan et al. 2005), resulting in greater quantities of plant protein in the landscape (increased nitrogen productivity). Tmax was found to be positively correlated with Tmin (r = 0.52, n = 30, p < 0.004) and negatively correlated with the amounts of summer rainfall (r = 0.54, n = 30, p < 0.003). Phases of relatively elevated Tmax therefore indicate conditions of increased solar radiation (reduced cloud cover) and reduced water availability for plant growth (reduced rainfall). The associated increased
photosynthesis-to-growth ratio is conducive to enhanced forage quality (increased storage allocation; reduced nitrogen dilution). During the initial phases of increasing temperature, Tmax is relatively high compared with Tmin and relatively high diurnal temperature ranges are thus encountered, supporting a pulse of enhanced SMP productivity (increased Tmax/Tmin ratio, increased Tmax – Tmin differential, and increased ratio of photosynthesis to respiration). This forms the basis of the first of four defined temperature acclimation phase states (SMP-P, phase of increased SMP productivity) constituting the temperature acclimation sequence resulting from (pulses of) increasing atmospheric temperature (Figure 6). However, as the Tmax/Tmin differential (diurnal temperature range; DTR) declines as nocturnal warming (Tmin)
increases faster than daytime temperatures, SMP-to-RMP mode acclimation occurs (decreased Tmax/Tmin ratio, decreased Tmax – Tmin differential, and increased ratio of respiration to photosynthesis) and sequential temperature acclimation phase states come into effect (Figure 6). The initially enhanced SMP mode productivity declines and RMP-mode growth increases [referred to as over-compensation by Wan et al. (2009)]. The associated shift from SMP-tW/Tw to RMP-TW functionality has physiological consequences which compromise forage quality (Figure 5), notably owing to a shift from storage-based to relative growth-dominated metabolism. This results in a reduction of stored metabolites (TNC, minerals and protein) relative to the accumulation of structural carbon through accelerated growth (reduced carbon-nutrient quality; Figure 5). Furthermore, RMP-TW mode functionality implies metabolic growth activity at
higher water and temperature optima. Growth realisation thus takes place under conditions of concurrently high temperature and water availability (TW conditions; at high water and temperature optima), constraining plant productivity under conditions when growth-curbed physiological activity (based on SMP-tw functional efficiency) associated with the production of high-quality forage (early morning hours, early in season, dry season growth: SMP-tw functionality) could have occurred. Dry-season rainfall supports SMP-tw functionality, thus promoting the production of forage items of high carbon-nutrient and nitrogen quality (Figure 5). However, with progressive SMP-to-RMP acclimation SMP-tw efficiency is expected to decline as RMP-TW functionality favours growth under conditions of higher temperature and water availability. SMP-to-RMP temperature acclimation (shifts from SMP-tW/Tw/tw to RMP-TW functionality) is therefore expected to result in forage with lower nitrogen quality (Figure 5: nitrogen dilution and lowered dry-season forage digestibility) and notably lower carbon-nutrient quality (Figure 5: low metabolic storage allocation priority).
 |
FIGURE 6: Sustained metabolic performance (SMP) to resource-responsive metabolic performance (RMP) mode temperature acclimation sequence involving four phase states: an initial SMP productivity pulse (SMP-t/SMP-T → SMP-P) followed by temperature acclimation (SMP-t/SMP-P → SMP-T → RMP-T) along the SMP-RMP acclimation trajectory (Figure 5) of intraspecific plant metabolic responses to temperature variables.
|
|
Spatiotemporal interactions in temperature acclimation and functionality settings: Following from the climate–vegetation response patterns postulated under the climate–vegetation response model we expect pulses of increased forage availability induced by pseudo-cyclic temperature increases and progressive long-term shifts towards increased RMP functionality settings.• Pulses of increased forage availability due to increased productivity of quality forage induced by pseudo-cyclic pulses of temperature increases (Figure 2 and 3: increasing towards 1940; increasing towards 1960; increasing after 1975): In response to transient rising Tmax, pulses of SMP productivity (SMP-P temperature acclimation phase state) occur when the temperature optima for plant functionality are still relatively low (tw metabolic activity). However, as acclimation to Tmin takes effect, increased RMP-TW metabolic activity of plants results in reductions in forage quality and thus forage availability (Figure 6). • Progressive long-term shifts towards relatively advanced RMP functionality settings in response to cumulative acclimation to Tmin and declining diurnal temperature ranges: Trends of progressively deteriorating carbon nutrient quality and increasing nitrogen productivity-to-nitrogen quality ratios (Figure 5) over the past century were accordingly anticipated (Figure 2). Owing to higher and more predictable levels of water availability to plants, such long-term shifts towards higher RMP functionality settings would have taken effect earlier (see Figures 2 and 3, notably in response to prolonged temperature increases between approximately 1930 and 1940–1945) and to more advanced RMP settings in the southern KNP (Table 1). Furthermore, as plants are already functioning at higher RMP settings in the southern KNP, SMP–RMP–SMP temperature response pulses (Figure 6) are expected to be attenuated relative to those in the northern KNP, where pronounced associated pulses of forage availability are expected to be encountered.
Model postulates and predictions
The postulates and predictions inferred from the climate–vegetation response model represent an explanatory framework geared to permit, inter alia, the interpretation of herbivore population performance patterns in space and over time. Based on the postulates of this model inferences can be made as to how the metabolic responses of plants to their dynamic edaphoclimatic environment shape forage availability for herbivores by affecting the levels of key forage resources (relating to nitrogen productivity, nitrogen quality and carbon-nutrient quality).
Key forage resources
The climate-vegetation response model differentiates three key indicators affecting forage availability to herbivores: nitrogen productivity, nitrogen quality and carbon-nutrient quality. Nitrogen productivity refers to the quantitative availability of forage items for herbivores of a given nitrogen quality (plant protein content) as a result of biomass production. High nitrogen quality of forage implies high nitrogen concentrations of plant tissues; that is, plant protein content not unduly diluted by structural or non-structural carbon and of relatively high digestibility. Carbon nutrient quality forage items are characterised by relatively high concentrations of non-structural carbohydrates and ash minerals resulting from metabolic allocation to storage under circumstances promoted by constrained growth (low temperatures, moisture stress: tw conditions), but sustained photosynthesis. Levels of these key forage resource indicators in plants (referring primarily to C4 grasses) are determined by plant metabolic responses to the spatiotemporally dynamic availability of resources (temperature, water and nutrients; Figure 5).
Herbivore species guilds of nutritional requirements
As inferred from the nature of the diets of eight large savanna herbivore species, three herbivore species guilds of nutritional requirements were identified: bulk feeders with tolerance to fibrous herbage (buffalo and waterbuck), bulk feeders with preference for high nitrogen quality forage (short-grass grazers: blue wildebeest and zebra) and selective feeders for which dietary items of relatively high carbon-nutrient quality represent key forage resources (selective grazers: sable antelope, roan antelope, tsessebe and eland).
Spatial distribution of key forage resources
Plant metabolic adjustments to the north–south water availability and altitudinal temperature gradients are expected to determine the spatial distribution of SMP to RMP functionality settings across the KNP (Table 1). The general pattern involved higher SMP functional expression in the drier, northern areas of the park and at higher altitudes, and advanced RMP functionality settings towards the south. As the SMP mode involves relatively growth-curbed, storage-based metabolism, the availability of forage of enhanced carbon-nutrient quality is accordingly expected to be higher in the northern KNP and at higher altitudes. Metabolic performance responses of intermediate SMP–RMP modes favour lower levels of nitrogen dilution and are associated with forage of relatively high nitrogen quality, especially of plants on nutrient-rich geological substrates (Table 1: central KNP).
Climate-driven temporal trends in forage availability
According to the climate–vegetation response model, both shorter-term transient temperature acclimation pulses (Figures 2 and 3: increasing temperature towards 1940; increasing towards 1960; increasing after 1975 ) and longer-term shifts towards advanced RMP functionality settings are predicted to have occurred in response to temperature trends over the past century (Figure 2). These temperature acclimation responses are expected to have resulted in transient pulses of increased forage availability (increased nitrogen and carbon-nutrient quality; Figure 6), as well as the progressive long-term decline of carbon-nutrient quality of forage and an increasing nitrogen productivity/nitrogen quality ratio associated with advanced RMP functionality settings (Figure 5).
Spatiotemporal interaction in forage availability
Accentuated temperature acclimation responses (Figure 6) are predicted for circumstances where low and unpredictable water availability favour the prevalence of SMP mode settings; that is, in northern relative to southern KNP. More pronounced associated effects on nitrogen quality and carbon-nutrient quality are thus expected towards the northern areas of the park, with attenuation or absence of such responses where relatively high RMP functionality settings already predominate towards the southern KNP.
The climate–vegetation response model as outlined here was applied as an explanatory framework with respect to herbivore population performance patterns in the KNP. Such application resulted in the internally consistent interpretation of spatiotemporal patterns of large herbivore population performance in relation to climate (Seydack et al. 2012).
We acknowledge the effort of rangers, other field staff and scientists in collecting diverse data sets (e.g. animal population censuses and grassland monitoring) over many decades, without which this study would not have been possible. Mss Judith Kruger, Sandra MacFadyen and Sharon Thomson of Scientific Services, KNP, are acknowledged for providing data used in this study. The South African Weather Service provided temperature and rainfall data. This study represents an output of the specialist scientist research programme in systems ecology conducted by members of the Conservation Services Division (Knysna and Skukuza) of South African National Parks. A.H.S, in his capacity as honorary research associate at the Botany Department, University of Cape Town, acknowledges general support received from this institution.
Competing interests
The authors declare that they have no financial or personal relationship(s) which may have inappropriately influenced them in writing this paper.
Authors’ contributions
A.H.S. (Garden Route National Park) was responsible for conceptualising and developing the climate–vegetation response model. C.C.G. (Kruger National Park), I.P.S. (Kruger National Park) and N.Z. (Kruger National Park) contributed to in-depth discussions through sharing their knowledge of animal and plant ecology pertaining to the KNP. W.J.V. (Garden Route National Park) acted as field project leader of the research into climate effects on forage quality and J.B. (Garden Route National Park) was involved in data processing.
An, Y., Wan, S., Zhou, Z., Subedar, A.A., Wallace, L.L. & Luo, Y., 2005, ‘Plant nitrogen concentration, use efficiency, and contents in a tallgrass prairie ecosystem under experimental
warming’, Global Change Biology 11, 1733–1744. http://dx.doi.org/10.1111/j.1365-2486.2005.01030.xBaas, W.J., 1989, ‘Secondary plant compounds, their ecological significance and consequences for the carbon budget. Introduction to the carbon/nutrient cycle theory’, in H. Lambers, M.L. Cambridge,
H. Konings & T.L. Pons (eds.), Causes and consequences of variation in growth rate and productivity of higher plants, pp. 313–340, S P B Academic Publishing, The Hague. Balasko, J.A. & Smith, D., 1971, ‘Influence of temperature and nitrogen fertilization on the growth and composition of switchgrass (Panicum virgatum L.) and timothy (Phleum pratense L.)
at anthesis’, Agronomy Jounal 63, 853–857.
http://dx.doi.org/10.2134/agronj1971.00021962006300060009x Bijoor, N.S., Czimczik, C.I., Pataki, D.E. & Billings, S.A., 2008, ‘Effects of temperature and fertilization on nitrogen cycling and community composition of an urban lawn’, Global
Change Biology 14, 2119–2131. http://dx.doi.org/10.1111/j.1365-2486.2008.01617.x Bodenstein, V., Meissner, H.H. & Van Hoven, W., 2000, ‘Food selection by Burchell’s zebra and blue wildebeest in the Timbavati area of the Northern Province Lowveld’,
South African Journal of Wildlife Research 30(2), 63–72. Buchmann, N., Brooks, J.R., Rapp, R.D. & Ehleringer, J.R., 1996, ‘Carbon isotope composition of C4 grasses is influenced by light and water’, Plant, Cell and Environment
19, 392–402. http://dx.doi.org/10.1111/j.1365-3040.1996.tb00331.x Codron, J., Codron, D., Lee-Thorp, J.A., Sponheimer, M., Bond, W.J., De Ruiter, D. et al., 2005, ‘Taxonomic, anatomical, and spatio-temporal variations in the stable carbon and nitrogen
isotopic compositions of plants from an African savanna’, Journal of Archaeological Science 32, 1757–1772.
http://dx.doi.org/10.1016/j.jas.2005.06.006 Dewar, R.C., Medlyn, B.E. & McMurtrie, R.E., 1999, ‘Acclimation of the respiration/photosynthesis ratio to temperature: Insights from a model’, Global Change Biology 5, 615–622.
http://dx.doi.org/10.1046/j.1365-2486.1999.00253.x Dörgeloh, W.G., 2006, ‘Habitat suitability for tsessebe Damaliscus lunatus lunatus’, African Journal of Ecology 44, 329–336.
http://dx.doi.org/10.1111/j.1365-2028.2006.00654.x Dunham, K.M., Robertson, E.F. & Grant, C.C., 2004, ‘Rainfall and the decline of a rare antelope, the tsessebe (Damaliscus lunatus lunatus), in Kruger National Park, South Africa’,
Biological Conservation 117, 83–94. http://dx.doi.org/10.1016/S0006-3207(03)00267-2 Dwyer, S.A., Ghannoum, O., Nicotra, A. & Von Caemmerer, S., 2007, ‘High temperature acclimation of C4 photosynthesis is linked to changes in photosynthetic biochemistry’,
Plant, Cell and Environment 30, 53–66. http://dx.doi.org/10.1111/j.1365-3040.2006.01605.x,
PMid:17177876
Ehleringer, J.R., Cerling, T.E. & Helliker, B.R., 1997, ‘C4 photosynthesis, atmospheric CO2, and climate’, Oecologia 112, 285–299.
http://dx.doi.org/10.1007/s004420050311 Engels, E.A.N. & Van der Merwe, F.J., 1967, ‘Application of an in vitro technique to South African forages with special reference to the effect of certain factors on the results’,
South African Journal of Agricultural Science 10, 983. Estiarte, M. & Peñuelas, J., 1999, ‘Excess carbon: the relationship with phenotypical plasticity in storage and defense functions of plants’, Orsis 14, 159–203. Fryxell, J.M., 1991, ‘Forage quality and aggregation by large herbivores’, American Naturalist 138(2), 478–498.
http://dx.doi.org/10.1086/285227 Fryxell, J.M., Wilmshurst, J.F., Sinclair, A.R.E., Haydon, D.T., Holt, R.D. & Abrams, P.A., 2005, ‘Landscape scale, heterogeneity, and the viability of Serengeti grazers’, Ecology
Letters 8, 328–335. http://dx.doi.org/10.1111/j.1461-0248.2005.00727.x Gertenbach, W., 1983, ‘Landscapes of the Kruger National Park’, Koedoe 26, 9–121. Grange, S. & Duncan, P., 2006, ‘Bottom-up and top-down processes in African ungulate communities: Resources and predation acting on the relative abundance of zebra and grazing bovids’,
Ecography 29, 899–907. http://dx.doi.org/10.1111/j.2006.0906-7590.04684.x Gureja, N. & Owen-Smith, N., 2002, ‘Comparative use of burnt grassland by rare antelope species in a lowveld game ranch, South Africa’, South African Journal of Wildlife Research
32(1), 31–38. Harrington, R., Owen-Smith, N., Viljoen, P.C., Mason, D.R. & Funston, P.J., 1999, ‘Establishing the causes of the roan antelope decline in the Kruger National Park, South Africa’, Biological
Conservation 90, 69–78. http://dx.doi.org/10.1016/S0006-3207(98)00120-7 Heitkönig, I.M.A. & Owen-Smith, N., 1998, ‘Seasonal selection of soil types and grass swards by roan antelope in a South African savanna’, African Journal of Ecology 36, 57–70.
http://dx.doi.org/10.1046/j.1365-2028.1998.114-89114.x Herms, D.A. & Mattson, W.J., 1992, ‘The dilemma of plants: To grow or defend’, Quarterly Review of Biology 67(3), 283–335.
http://dx.doi.org/10.1086/417659 Hofmann, R.R., 1973, The ruminant stomach: Stomach structure and feeding habits of east african game ruminants, Vol. II, East African Literature Bureau, Nairobi. Hofmann, R.R., 1989, ‘Evolutionary steps of ecophysiological adaptation and diversification of ruminants: a comparative view of their digestive systems’, Oecologia 78, 443–457.
http://dx.doi.org/10.1007/BF00378733 Holdo, R.M., Holt, R.D. & Fryxell, J.M., 2009, ‘Opposing rainfall and plant nutritional gradients best explain the wildebeest migration in the Serengeti’, American Naturalist 173(4), 431–445.
http://dx.doi.org/10.1086/597229,
PMid:19243258
Houghton, J.T., Meira Filho, L.G., Callander, B.A., Harris, N., Kattenberg, A. & Maskell, K. (eds.), 1996, Climate Change 1995: The Science of Climate Change, Cambridge University Press, Cambridge. Joubert, S.C.J., 1976, ‘The population ecology of the roan antelope (Hippotragus equinus equinus) (Desmarest, 1804) in the Kruger National Park’, DSc thesis, Faculty of Science, University of Pretoria. Joubert, S.C.J. & Bronkhorst, P.H.L., 1977, ‘Some aspects of the history and population ecology of the Tsessebe Damaliscus lunatus lunatus in the Kruger National Park’, Koedoe 20, 125–145. Joubert, S., 2007a, The Kruger National Park: A history, Vol. I, High Branching, Johannesburg. Joubert, S., 2007b, The Kruger National Park: A history, Vol. II., High Branching, Johannesburg. Kennedy, A.D., Biggs, H. & Zambatis, N., 2003, ‘Relationship between grass species richness and ecosystem stability in Kruger National Park, South Africa’, African Journal of Ecology
41, 131–140. http://dx.doi.org/10.1046/j.1365-2028.2003.00391.x Lambers, H. & Poorter, H., 1992, ‘Inherent variation in growth rate between higher plants: a search for physiological causes and ecological consequences’, Advances in
Ecological Research 23, 187–261. http://dx.doi.org/10.1016/S0065-2504(08)60148-8 Lambers, H., Chapin III, F.S. & Pons, T.L., 1998, Plant Physiological Ecology, Springer Verlag, Berlin. Le Roux, E., 2010, ‘Habitat and forage dependency of sable antelope (Hippotragus niger) in the Pretoriuskop region of the Kruger National Park’, MSc thesis, Faculty of Science,
University of the Witwatersrand. Long, S.P., 1999, ‘Environmental responses’, in R.F. Sage & R.K. Monson (eds.), C4 Plant Biology, pp. 215–249, Academic Press, San Diego.
http://dx.doi.org/10.1016/B978-012614440-6/50008-2 Luo, Y., 2007, ‘Terrestrial carbon-cycle feedback to climate warming’, Annual Review of Ecology, Evolution and Systematics 38, 683–712.
http://dx.doi.org/10.1146/annurev.ecolsys.38.091206.095808 Macandza, V.A., Owen-Smith, N. & Cross, P.C., 2004, ‘Forage selection by African buffalo in the late dry season in two landscapes’, South African Journal of Wildlife Research 34(2), 113–121. Magome, D.T., 1991, ‘Habitat selection and feeding ecology of the sable antelope, Hippotragus niger niger (Harris 1838), in Pilanesberg National Park, Bophuthatswana’, MSc thesis, Faculty of
Science, University of the Witwatersrand. Magome, H., Cain, J.W.I., Owen-Smith, N. & Henley, S.R., 2008, ‘Forage selection of sable antelope in Pilanesberg Game Reserve, South Africa’, South African Journal of Wildlife Research
38(1), 35–41. http://dx.doi.org/10.3957/0379-4369-38.1.35 ‘t Mannetje, L., 1975, ‘Effect of daylength and temperature on introduced legumes and grasses for the tropics and subtropics of coastal Australia. 2. N-concentration, estimated digestibility
and leafiness’, Australian Journal of Experimental Agriculture and Animal Husbandry 15, 256–263. http://dx.doi.org/10.1071/EA9750256 ‘t Mannetje, L., 1982, ‘Problems of animal production from tropical pastures’, in J.B. Hacker (ed.), Nutritional limits to animal production from pastures, pp. 67–85,
Commonwealth Agricultural Bureaux, Farnham Royal. ‘t Mannetje, L, 1984, ‘Nutritive value of tropical and subtropical pastures, with special reference to protein and energy deficiency in relation to animal production’, in F.M.C.
Gilchrist & R.I. Mackie (eds.), Herbivore nutrition in the subtropics and tropics, pp. 56–66, The Science Press, Craighall. Mutanga, O., Prins, H.H.T., Skidmore, A.K., Van Wieren, S., Huizing, H., Grant, R. et al., 2004, ’Explaining grass-nutrient patterns in a savanna rangeland of southern Africa’, Journal
of Biogeography 31, 819–829. http://dx.doi.org/10.1111/j.1365-2699.2004.01072.x Osborne, C.P., 2008, ‘Atmosphere, ecology and evolution: what drove the Miocene expansion of C4 grasslands?’, Journal of Ecology 96, 35–45.
PMid:18784799,
PMCid:2517376
Owen-Smith, N. & Ogutu, J.O., 2003, ‘Rainfall influences on ungulate population dynamics’, in J.T. du Toit, H.C. Biggs & K.H. Rogers (eds.), The Kruger experience: Ecology and
management of savanna heterogeneity, pp. 310–331, Island Press, Washington DC. Owen-Smith, N., Mason, D.R. & Ogutu, J.O., 2005, ‘Correlates of survival rates of ten African ungulate populations: Density, rainfall and predation’, Journal of Animal Ecology 74,
774–778. http://dx.doi.org/10.1111/j.1365-2656.2005.00974.x Owen-Smith, N. & Mills, M.G.L., 2006, ‘Manifold interactive influences on the population dynamics of a multispecies ungulate assemblage’, Ecological Monographs 76, 73–92.
http://dx.doi.org/10.1890/04-1101 Owen-Smith, N. & Mills, M.G.L., 2008, ‘Shifting prey selection generates contrasting herbivore dynamics within a large-mammal predator-prey web’, Ecology 89(4), 1120–1133.
http://dx.doi.org/10.1890/07-0970.1,
PMid:18481536
Parrini, F., 2006, ‘Nutritional and social ecology of the sable antelope in a Magaliesberg Nature Reserve’, PhD thesis, School of Animal, Plant and Environmental Sciences, University of the
Witwatersrand. Pienaar, U de V., 1963, ‘The large mammals of the Kruger National Park – their distribution and present-day status’, Koedoe 6, 1–37. Schulze, R.E., 1995, Hydrology and agrohydrology: A text to accompany the ACRU 3.00 agro-hydrological modeling system, Department of Agricultural Engineering, University of Natal, Pietermartizburg. Schulze, R.E., 1997, South African atlas of agrohydrology and –climatology, Department of Agricultural Engineering, University of Natal, Pietermaritzburg. Seydack, A.H., Grant, C.C., Smit, I.P., Vermeulen, W.J., Baard, J. & Zambatis, N., 2012, ‘Large herbivore population performance and climate in a South African semi-arid savanna’, Koedoe 54(1), Art. #1047, 20 pages.
http://dx.doi.org/10.4102/koedoe.v54i1.1047 Skidmore, A.K., Ferwerda, J.G., Mutanga, O., Van Wieren, S.E., Peel, M., Grant, R.C. et al., 2010, ‘Forage quality of savannas: Simultaneously mapping foliar protein and polyphenols for
trees and grass using hyperspectral imagery’, Remote Sensing of Environment 114, 64–72.
http://dx.doi.org/10.1016/j.rse.2009.08.010 Skinner, J.D. & Chimimba, C.T., 2005, The Mammals of the Southern African Subregion, 3rd edn., Cambridge University Press, Cambridge. Smuts, G.L., 1972, ‘Seasonal movements, migration and age determination of Burchell’s zebra (Equus burchelli antiquorum, H. Smith, 1841) in the Kruger National Park’, MSc thesis,
Faculty of Science, University of Pretoria. Taylor, G.J., 1989, ‘Maximum potential growth rate and allocation of respiratory energy to stress tolerance in plants’, Plant Physiology and Biochemistry 27, 605–611. Tilley, J.M.A. & Terry, R.A., 1963, ‘A two-stage technique for the in vitro digestion of forage crops’, Journal of the British Grassland Society 18, 104–111.
http://dx.doi.org/10.1111/j.1365-2494.1963.tb00335.x Traill, L.W., 2004, ‘Seasonal utilization of habitat by large grazing herbivores in semi-arid Zimbabwe’, South African Journal of Wildlife Research 34(1), 13–24. Tudsri, S.H., Matsuoka, H. & Kobashi, K., 2002, ‘Effect of temperature on seedling growth characteristics of Panicum maximum’, Tropical Grasslands 36, 165–171. Van Lavieren, L.P. & Esser, J.D., 1979, ‘Numbers, distribution and habitat preference of large mammals in Bouba Ndjida National Park, Cameroon’, African Journal of Ecology 17, 141–153. Van Soest, P.H., Mertens, D.R. & Deinum, B., 1978, ‘Preharvest factors influencing quality of conserved forage’, Journal of Animal Science 47, 712–720. Venter, F.J., Scholes, R.J. & Eckhardt, H.C., 2003, ‘The abiotic template and its associated vegetation patterns’, in J.T. du Toit, K.H. Rogers & H.C. Biggs (eds.), The Kruger experience:
Ecology and management of savanna heterogeneity, pp. 83–129, Island Press, Washington DC. Wan, S., Hui, D.F., Wallace, L.L. & Luo, Y., 2005, ‘Direct and indirect effects of experimental warming on ecosystem carbon processes in a tallgrass prairie’, Global Biogeochemical Cycles
19, GB2014.
http://dx.doi.org/10.1029/2004GB002315
Wan, S., Xia, J., Liu, W. & Niu, S., 2009, ‘Photosynthetic overcompensation under nocturnal warming enhances grassland carbon sequestration’, Ecology 90(10), 2700–2710.
http://dx.doi.org/10.1890/08-2026.1,
PMid:19886480
Watson, L.H. & Owen-Smith, N., 2000, ‘Diet composition and habitat selection of eland in semi-arid shrubland’, African Journal of Ecology 38, 130–137.
http://dx.doi.org/10.1046/j.1365-2028.2000.00229.x Whyte, I.J. & Joubert, S.C.J., 1988, ‘Blue wildebeest population trends in the Kruger National Park and the effect of fencing’, South African Journal of Wildlife Research 18(3), 78–87. Wilson, J.R., 1975, ‘Influence of temperature and nitrogen on growth, photosynthesis and accumulation of non-structural carbohydrate in a tropical grass, Panicum maximum var. trichoglume’,
Netherlands Journal of Agricultural Science 23, 48–61. Wilson, J.R., 1982, ‘Environmental and nutritional factors affecting herbage quality’, in J.B. Hacker (ed.), Nutritional limits to animal production from pastures, pp. 111–131, Commonwealth
Agricultural Bureaux, Farnham Royal. Wilson, J.R. & Ford, C.W., 1971, ‘Temperature influences on the growth, digestibility, and carbohydrate composition of two tropical grasses, Panicum maximum var. trichoglume and Setaria
sphacelata, and two cultivars of the temperate grass Lolium perenne’, Australian Journal of Agricultural Research 22, 563–571.
http://dx.doi.org/10.1071/AR9710563 Wilson, J.R. & Ford, W., 1973, ‘Temperature influences on the in vitro digestibility and soluble carbohydrate accumulation of tropical and temperate grasses’, Australian Journal of
Agricultural Research 24, 187–198. http://dx.doi.org/10.1071/AR9730187 Wilson, J.R., Taylor, A.O. & Dolby, G.R., 1976, ‘Temperature and atmospheric humidity effects on cell wall content and dry matter digestibility of some tropical and temperate grasses’,
New Zealand Journal of Agricultural Research 19, 41–46. Wilson, J.R. & ‘t Mannetje, L., 1978, ‘Senescence, digestibility and carbohydrate content of buffel grass and green panic leaves in swards’, Australian Journal of Agricultural
Research 29, 503–516. http://dx.doi.org/10.1071/AR9780503 Zambatis, N., 2004, ‘Summary report of trends in herbaceous veld condition in the ranger sections of the Kruger National Park during the period 1989 to 2004’, Scientific Services, Kruger National Park, Skukuza.
|